Strides Recall Several Lots of Topical G
Strides Pharma Inc is recalling over 38 lots of Testosterone Gel 1% in the US

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Strides Pharma Inc is recalling over 38 lots of Testosterone Gel 1% in the US

Glenmark Pharmaceuticals has initiated a recall of over 1.5 million bottles of their ADHD medication,
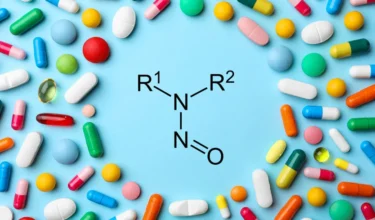
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Adding to the growing number of drug recalls due to NDSRIs in 2024, Glenmark is

Adding to the number of recalls during 2024 for NDSRIs, Ascend (Alkem) is recalling the
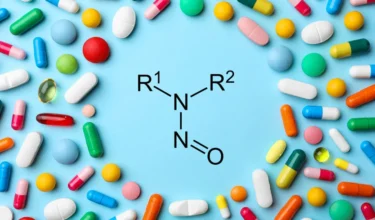
Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has

Several lots of the antidepressant drug Duloxetine, manufactured by Aurobindo, India, have been recalled in

Authored by: Venkiteswaran.T.K, Subrangshu Chourdhary, Satish Reddy Lupin is recalling over 600,000 bottles of Ramipril capsules across different

New Delhi based Unexo Life Sciences has initiated a major recall of several medicated patch

Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has