

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations
Glenmark Pharmaceuticals has initiated a recall of over 1.5 million bottles of their ADHD medication, Atomoxetine, across the US and Europe due to the presence of the nitrosamine impurity N-Nitroso atomoxetine. This marks the first recall of Atomoxetine for the presence of the Nitrosamine Drug Substance Related Impurity (NDSRI).
Atomoxetine is indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). Glenmark’s recall encompasses multiple strengths of Atomoxetine capsules, including 10, 18, 25, 40, 60, 80, and 100 mg doses. The recall was initiated in the US in January 2025, followed by similar actions in Europe as per safety notice release by the Health Products Regulatory Authority (HPRA) of Ireland.
As per EMA (European Medicine Agency (EMA), the N-Nitroso atomoxetine impurity has a maximum acceptable intake limit of 100 ng/day, established through the Structure Activity Relationship (SAR) approach. (Appendix 1: Acceptable intakes established for N-nitrosamines; Questions and Answers on nitrosamine impurities in human medicinal products). The USFDA CDER Nitrosamine Impurity acceptable intake limits database has not prescribed any limit for the impurity.
Nitrosamines, including NDSRIs, are impurities formed when secondary or tertiary amines react with nitrosating agents, typically nitrite impurities during the API synthesis or the manufacture of the drug product. Nitrosamines and NDSRI impurities in medicinal products are a concern due to their potential carcinogenicity, properties and considered cohort of concern. Atomoxetine has a reactive secondary amine group which can react with nitrosating agents to give N-Nitroso atomoxetine impurity.
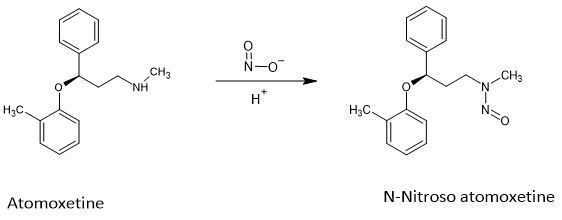
Atomoxetine was initially approved in US in 2002 and has several generics in the US including from companies Glenmark, Teva, Aurobindo, Strides, Dr.Reddys, Apotex among others. However this is the first instance of a recall of Atomoxetine due to the presence of the NDSRI N-Nitroso atomoxetine
Formation of N-Nitroso atomoxetine
Different synthetic routes are discussed in the literature for synthesis of atomoxetine, none of which require involvement of a nitrosating agent like sodium nitrite.
Given that none of these methods involve nitrosating agents, the likelihood of N-Nitroso atomoxetine formation through these synthetic pathways is minimal.
Manufacture of atomoxetine capsules involve excipients like gelatin, titanium dioxide, pregelatinized starch, sodium lauryl sulfate. Azo dyes like FD&C Yellow No. 6, FD&C Red No. 40 are used as colouring agents in the capsule shells and / or imprinting ink. Excipients like pregelatinized starch can contain nitrite impurities, which, during the manufacturing process, can react with the secondary amine group in Atomoxetine to form N-Nitroso atomoxetine. Additionally, azo dyes, synthesized through diazotization reactions involving sodium nitrite, may carry nitrite impurities. Although the quantities of these coloring agents used in the formulation are minimal, their potential contribution to nitrosamine impurity formation cannot be ignored and requires monitoring and control.
Control of NDSRI Formation in Drug Products.
Given the increasing number of NDSRI-related recalls, and new recalls of drug products for NDSRIs not reported hitherto, its imperative that there should be a robust risk assessment and control strategies for excipients to manage nitrosating impurities. Where actives (APIs) in the drug product has a vulnerable functional group susceptible to forming nitrosamine impurities it become crucial to:
References

For More On Nitrosamines and NDSRIs
Leave a Comment
You must be logged in to post a comment.