Nitrosamine Impurities in Pharmaceutical
Authored by: Venkiteswaran.T.K Nitrosamine impurities in pharmaceutical products are a significant concern due to their

Warning letters, 483s, Recalls, Import Alerts, Audit observations
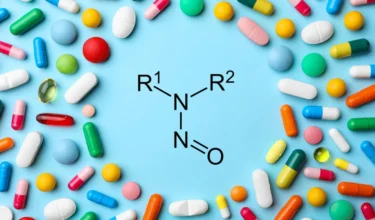
Authored by: Venkiteswaran.T.K Nitrosamine impurities in pharmaceutical products are a significant concern due to their
As per EUGMP requirements, EudraLex, Annex 16: Certification by a Qualified Person and Batch Release,
Author: Venkiteswaran.T.K The USFDA’s 2011 guidance on process validation advocates a life cycle approach and

In March 2025, Glenmark initiated recall of 23 products (148 lots) in US for cGMP

According to the EU GMP guidance—“Guidance for Risk Assessment for Ascertaining the Appropriate Good Manufacturing

Authored by: Srinivas Churya This Quality Insight post presents a concise approach for selection and

Piramal Pharma’s API unit at Navi Mumbai (FEI 3008763768) was issued USFDA Form 483 with

Granules India received a warning letter from the USFDA in February 2025. This was along

Janssen’s Korean Vaccine unit Janssen Vaccines Corporation (FEI 3012637764) received an USFDA 483 for critical

Indian API manufacturer Jagsonpal Pharmaceuticals was issued a Warning Letter by the US FDA in