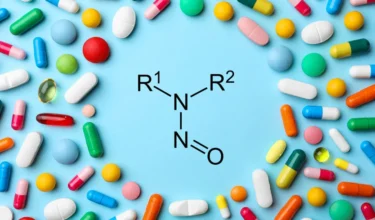
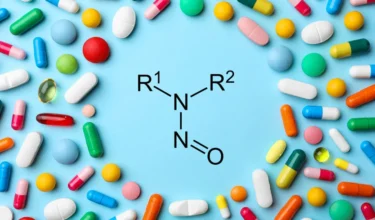
Concerns of Beta-lactam Contamination Tr
In March 2025, Glenmark initiated recall of 23 products (148 lots) in US for cGMP

Warning letters, 483s, Recalls, Import Alerts, Audit observations

In March 2025, Glenmark initiated recall of 23 products (148 lots) in US for cGMP
With timelines closing in for drug product manufacturers to finalize their nitrosamine risk assessments, recalls
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Adding to the number of recalls during 2024 for NDSRIs, Ascend (Alkem) is recalling the

Authored by: Venkiteswaran.T.K, Subrangshu Chourdhary, Satish Reddy Lupin is recalling over 600,000 bottles of Ramipril capsules across different
In October 2024, Accord Healthcare recalled over 25 lots of Cinacalcet tablets in various strengths

Dr.Reddys Laboratories (DRL) has initiated recall of one lot of Allopurinol Tablets, USP 300mg in

Sagent is recalling two lots of Docetaxel injection in US. The recall follows a customer

SCYNEXIS recalled 2 lots of Brexafemme (ibrexafungerp) tablets in U.S. due to potential cross contamination

Macleods recalled 10052 bottles of Levofloxacin USP 500mg tablets in January 2023 due to mismatching