Chattem Inc Recall Antihistamine Sleepin
With timelines closing in for drug product manufacturers to finalize their nitrosamine risk assessments, recalls

Warning letters, 483s, Recalls, Import Alerts, Audit observations
With timelines closing in for drug product manufacturers to finalize their nitrosamine risk assessments, recalls

Piramal Pharma’s API unit at Navi Mumbai (FEI 3008763768) was issued USFDA Form 483 with

The FDA inspected Colgate-Palmolive/Tom’s of Maine, Inc’s Sanford facility in the US in May 2024.
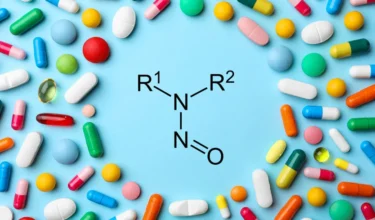
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Authored by: Venkiteswaran.T.K, Srinivas Churya FDA has published the Warning Letter issued to the Viatris Pithampur facility in December

Adding to the number of recalls during 2024 for NDSRIs, Ascend (Alkem) is recalling the
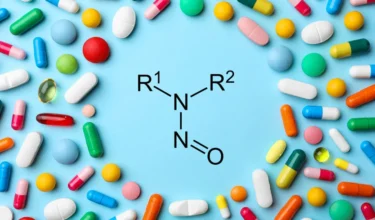
Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has

Authored by: Venkiteswaran.T.K, SubrangshuChourdhary, Srinivas Churya, Satish Reddy, Sanjeev Kumar Singh Aarti Drugs API facility in Tarapur, India

Authored by: Venkiteswaran.T.K, Subrangshu Chourdhary, Satish Reddy Lupin is recalling over 600,000 bottles of Ramipril capsules across different

Authored by: Venkiteswaran.T.K, Subrangshu Chourdhary, Srinivas Churya Jubilant Generics Roorkee facility was inspected in January