Unichem Recalls Hypertension Tablets for
Unichem Pharmaceuticals (USA), Inc. initiated a recall of Bisoprolol fumarate and Hydrochlorothiazide tablets in the

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Unichem Pharmaceuticals (USA), Inc. initiated a recall of Bisoprolol fumarate and Hydrochlorothiazide tablets in the

Imprimis NJOF recalled three intraocular ophthalmic injection drug products in December 2025 (as per USFDA
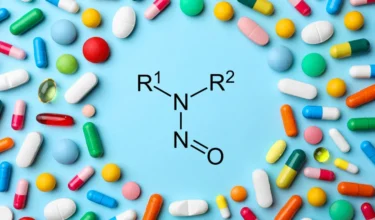
Zydus is recalling multiple lots of the antipsychotic drug Chlorpromazine in U.S due to the
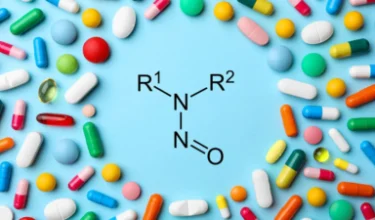
Alembic recently initiated recall of antidepressant drug Doxepin Hydrochloride Capsules (10 mg) in US for Nitrosamine

Accord (Intas) initiated a recall of several lots of Levothyroxine tablets in the US for

In March 2025, Glenmark initiated recall of 23 products (148 lots) in US for cGMP

First Recall of Diphenhydramine HCl for NDSRI With timelines closing in for drug product manufacturers
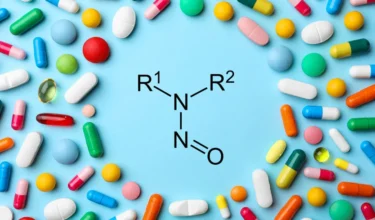
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Adding to the number of recalls during 2024 for NDSRIs, Ascend (Alkem) is recalling the

Authored by: Venkiteswaran.T.K, Subrangshu Chourdhary, Satish Reddy Lupin is recalling over 600,000 bottles of Ramipril capsules across different