Data Integrity, Cleaning Issues: Granule
USFDA inspected Granules India’s Finished Dosage and PFI facility (FEI 3004097901) at Gagillapur Village, Hyderabad

Warning letters, 483s, Recalls, Import Alerts, Audit observations

USFDA inspected Granules India’s Finished Dosage and PFI facility (FEI 3004097901) at Gagillapur Village, Hyderabad
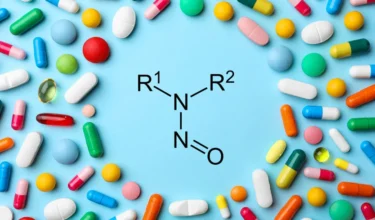
In October 2024, Accord Healthcare recalled over 25 lots of Cinacalcet tablets in various strengths

Authored by: Srinivas Churya In the pharmaceutical industry, analytical division and analytical laboratories plays a

Five different facilities of Eugia across India and US were inspected by USFDA between December

With contributions from: Venkiteswaran.T.K; Satish Reddy; Shashank Sharma; Srinivas Churya; Subrangshu Choudhary The USFDA inspection

With contributions from: Venkiteswaran.T.K; Satish Reddy; Shashank Sharma; Srinivas Churya; Subrangshu Choudhary, Veena Raj The USFDA Form

The Eugia facility in Bhiwadi, Rajasthan, India (FEI 3009883410) was recently classified as OAI (Official

Biocon Visakh (Biocon Biosphere Limited, FEI 30221 25340) was inspected by USFDA investigator Brandy N

Zydus Jarod facility (FEI 3013712903) is classified OAI by USFDA in July 2024. The USFDA

Sun Pharma’s Dadra Unit was issued FDA Warning letter in June 2024, following critical observations