Nitrosocinacalcet Nitrosamine Impurity T
In October 2024, Accord Healthcare recalled over 25 lots of Cinacalcet tablets in various strengths

Warning letters, 483s, Recalls, Import Alerts, Audit observations
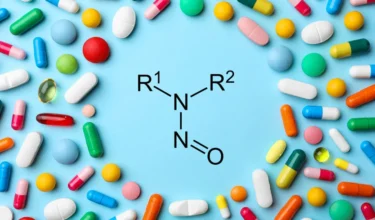
In October 2024, Accord Healthcare recalled over 25 lots of Cinacalcet tablets in various strengths

Accord Healthcare Inc, US is recalling one lot of Cisplatin injection due to failed impurities.

CDSCO has published a drug alert regarding voluntary recall of Tisseel Lyo Fibrin Sealant VHS/D

Dr.Reddy’s laboratories Inc (DRL) has initiated recall of 50 batches of Ibuprofen tablets in US

Zydus Pharmaceuticals (USA) Inc initiated recall of multiple lots of Verapamil Hydrochloride injection due to

Dr.Reddys Laboratories (DRL) has initiated recall of one lot of Allopurinol Tablets, USP 300mg in

Glenmark Pharmaceuticals is recalling 114 batches of Potassium Chloride Extended-Release Capsules, USP (750 mg) 10

Eugia US LLC is recalling two more injectable products in US for failed impurities, degradation
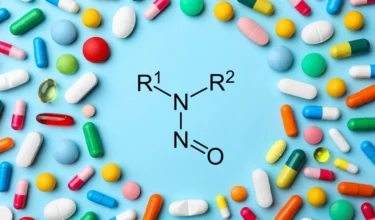
Glenmark recalled several lots of Rizatriptan Benzoate Tablets and Rizatriptan Benzoate Orally Disintegrating Tablets in

Sagent is recalling two lots of Docetaxel injection in US. The recall follows a customer