FDA Extends Nitrosamine Timelines
USFDA has allowed drug manufacturers additional time for submission of mitigation actions and required changes

Warning letters, 483s, Recalls, Import Alerts, Audit observations
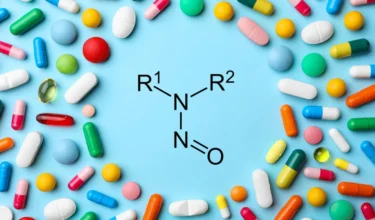
USFDA has allowed drug manufacturers additional time for submission of mitigation actions and required changes

Authored by: Venkiteswaran.T.K Nitrosamine impurities in pharmaceutical products are a significant concern due to their

Breckenridge Pharmaceutical Inc. has announced the recall of over 360,000 bottles of Duloxetine Delayed-Release Capsules

WHO has published a comprehensive and easy to understand guideline for control of nitrosamines in
With timelines closing in for drug product manufacturers to finalize their nitrosamine risk assessments, recalls

Glenmark Pharmaceuticals has initiated a recall of over 1.5 million bottles of their ADHD medication,
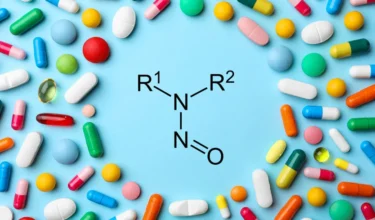
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Adding to the growing number of drug recalls due to NDSRIs in 2024, Glenmark is

Adding to the number of recalls during 2024 for NDSRIs, Ascend (Alkem) is recalling the
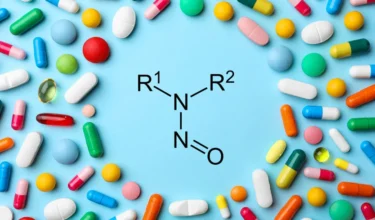
Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has