Unichem Recalls Hypertension Tablets for
Unichem Pharmaceuticals (USA), Inc. initiated a recall of Bisoprolol fumarate and Hydrochlorothiazide tablets in the

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Unichem Pharmaceuticals (USA), Inc. initiated a recall of Bisoprolol fumarate and Hydrochlorothiazide tablets in the

Qvents provides useful tools for QMS processes – Risk Assessments, Continuous Process Validations, Supply Chain Traceability
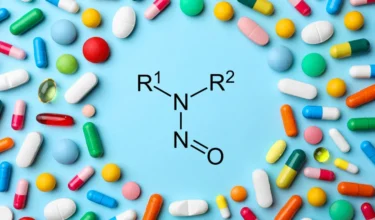
Zydus is recalling multiple lots of the antipsychotic drug Chlorpromazine in U.S due to the
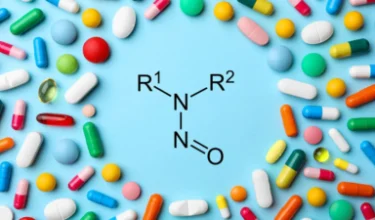
Alembic recently initiated recall of antidepressant drug Doxepin Hydrochloride Capsules (10 mg) in US for Nitrosamine
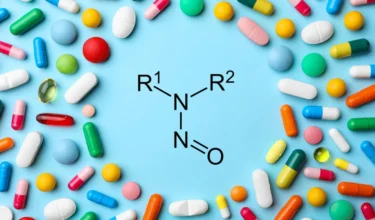
USFDA has allowed drug manufacturers additional time for submission of mitigation actions and required changes

Qvents Apps & ToolsNitrosamine and NDSRI Risk Assessment Tools – APIs, Drug Products, Excipients, Water

Breckenridge Pharmaceutical Inc. has announced the recall of over 360,000 bottles of Duloxetine Delayed-Release Capsules

WHO has published a comprehensive and easy to understand guideline for control of nitrosamines in

First Recall of Diphenhydramine HCl for NDSRI With timelines closing in for drug product manufacturers

Glenmark Pharmaceuticals has initiated a recall of over 1.5 million bottles of their ADHD medication,