USFDA issues new draft guidance for Post
As per the GDUFA III commitment letter, USFDA has issued a draft guidance for facilities

Warning letters, 483s, Recalls, Import Alerts, Audit observations

As per the GDUFA III commitment letter, USFDA has issued a draft guidance for facilities
The USFDA Warning letter to K. C. Pharmaceuticals issued in August 2023, following inspection at

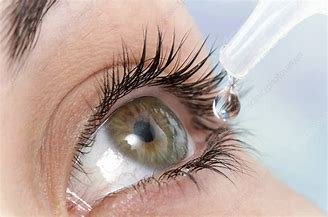
In a Class III recall in US, Alembic recalled 82400 bottles of Tobramycin 0.3%, Ophthalmic

Rufinamide tablets are indicated for adjunctive treatment of seizures associated with Lennox-Gastaut Syndrome (LGS). Aurobindo

Biocon Malaysia was inspected by USFDA Investigators Eileen A. Liu, Patty P. Kaewussdangkul, Daniel Lahar,

Several manufacturers of OTC drug products including mouthwash, toothpaste, topical analgesics, Sunscreen products, Hand Sanitizers

USFDA: Recommended Acceptable Intake Limits for Nitrosamine Drug Substance[1]Related Impurities (NDSRIs) – Guidance for Industry

Zurzuvae (zuranolone), the first oral medication to treat postpartum depression (PPD) in adults. PPD is a

The GDUFA fee rates show steepest increase for DMF by 21% to $94,682 from $78,293.

Related Links Warning letter Intas Warning letter Centaur Warning letter Medgel Warning letter Baxter Intas