

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations
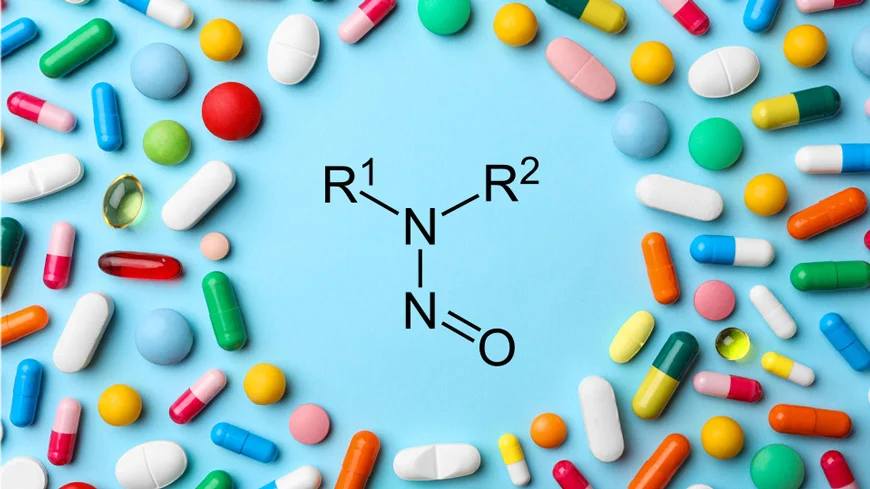
EMA(Europeans Medicines Agency) has updated the Appendix 1 Acceptable intakes established for N-nitrosamines with 15 more substances in February 2024. The new list cover drug molecules Cyanocobalamine, Apixaban, Clozapine, Tamoxifen, Flecainide, Masitinib, Telmisartan, Meropenem, Methylphenidate, Silodosin and Terazosin. The list has now 133 N-Nitrosamines (NDSRIs).
EMA has published Questions and answers (Q&A) for marketing authorization holders/applicants on implementing the CHMP opinion on Nitrosamine impurities in human medicinal products which gets updated regularly. The Q&A on CHMP opinion on Nitrosamine impurities require the Marketing Authorisation (MA) holders of human medicinal products to ensure nitrosamines are controlled and kept as low as possible in the medicinal products. The MA holders shall assess risk of nitrosamines in the products considering manufacturing processes including API and materials (excipients) used, inform authorities outcome of the risk assessment, perform confirmatory testing when risk is identified, define limits for the Nitrosamine impurities following the CPCA approach or substance specific limit, report results, implement necessary controls for nitrosamine impurities and update the MA for measures taken to prevent or minimise risk of nitrosamines in human medicinal products. The Appendix 1 of the Q&A document lists Acceptable Intakes (Ais) for N-Nitrosamine impurities in medicinal products.
Related links: EMA/CMDh: Appendix 1 for Nitrosamines Updated Again
Leave a Comment
You must be logged in to post a comment.