FDA Extends Nitrosamine Timelines
USFDA has allowed drug manufacturers additional time for submission of mitigation actions and required changes

Warning letters, 483s, Recalls, Import Alerts, Audit observations
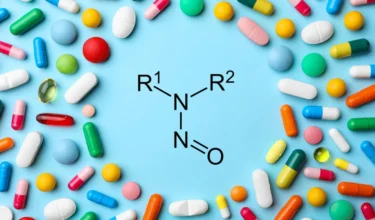
USFDA has allowed drug manufacturers additional time for submission of mitigation actions and required changes

USFDA has issued the final guidance for Pre-Submission Facility Correspondence (PFC) for prioritised generic drug

Indian regulatory agency, CDSCO has updated the requirements for application and issuance of Export NOC

The Drugs Technical Advisory Board (DTAB) in India has recommended for amendment of Drug Rules

WHO has published a comprehensive and easy to understand guideline for control of nitrosamines in

Indian drug regulatory, CDSCO has notified the new online Export NOC system for Unapproved Drugs

Health Ministry in India has extended the timeline for implementation of the revised Schedule M

The FDA has issued new guidance on “Considerations for Complying With 21 CFR 211.110.” According

Chinese OTC Manufacturer Tianjin Darentang Jingwanhong Pharmaceutical Co., Ltd was inspected by USFDA in March

The USFDA has published a new guidance titled “Review of Drug Master Files in Advance