Glenmark Warning Letter Cites 21 CFR 211
USFDA issued a Warning letter to Glenmark Facility at Pithampur (FEI 3008565058) in July 2025.

Warning letters, 483s, Recalls, Import Alerts, Audit observations

USFDA issued a Warning letter to Glenmark Facility at Pithampur (FEI 3008565058) in July 2025.

Natco Pharma’s sterile products manufacturing facility at Rangareddy, Telengana (FEI 3004540906) was inspected by USFDA

Accord (Intas) initiated a recall of several lots of Levothyroxine tablets in the US for
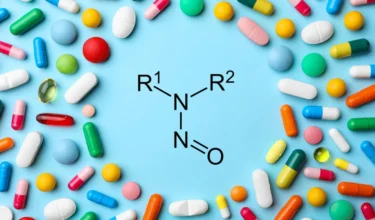
Qvents Apps & ToolsNitrosamine and NDSRI Risk Assessment Tools – APIs, Drug Products, Excipients, Water
As per EUGMP requirements, EudraLex, Annex 16: Certification by a Qualified Person and Batch Release,
Author: Venkiteswaran.T.K The USFDA’s 2011 guidance on process validation advocates a life cycle approach and

In March 2025, Glenmark initiated recall of 23 products (148 lots) in US for cGMP

Qvents Apps & Tools Access the Excipient Risk Assessment Tool – Click Here According to

Authored by: Srinivas Churya This Quality Insight post presents a concise approach for selection and

Piramal Pharma’s API unit at Navi Mumbai (FEI 3008763768) was issued USFDA Form 483 with