NDSRIs: Glenmark Recalls Several Lots of
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Warning letters, 483s, Recalls, Import Alerts, Audit observations
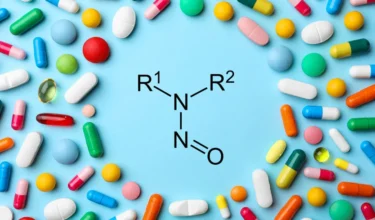
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Adding to the growing number of drug recalls due to NDSRIs in 2024, Glenmark is

Adding to the number of recalls during 2024 for NDSRIs, Ascend (Alkem) is recalling the
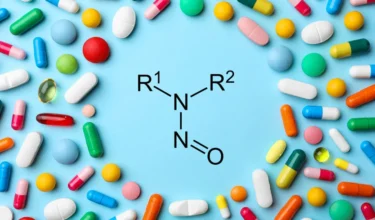
Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has

Several lots of the antidepressant drug Duloxetine, manufactured by Aurobindo, India, have been recalled in

Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has

In October 2024, Accord Healthcare recalled over 25 lots of Cinacalcet tablets in various strengths

FDA has issued an Updated Guidance for Control of Nitrosamines in Human Drugs in September
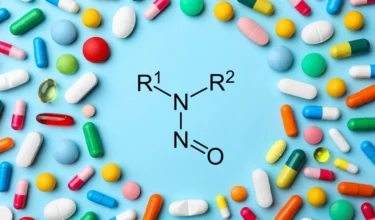
Glenmark recalled several lots of Rizatriptan Benzoate Tablets and Rizatriptan Benzoate Orally Disintegrating Tablets in
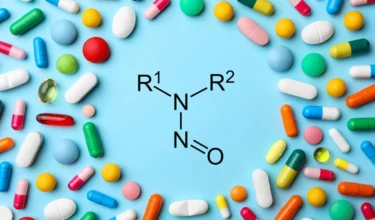
EMA(Europeans Medicines Agency) has updated the Appendix 1 Acceptable intakes established for N-nitrosamines with 15