

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Several lots of the antidepressant drug Duloxetine, manufactured by Aurobindo, India, have been recalled in the US due to the nitrosamine impurity NDSRI, N-Nitroso-duloxetine. More than 600K bottles of Duloxetine Capsules across strengths of 20mg, 30mg, and 60mg were recalled by repackagers/distributors Rising Pharma and Amerisource Health Services LLC. The batches are being recalled because the nitrosamine (NDSRI) impurity N-Nitroso-duloxetine is above acceptable limits. According to the USFDA (Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) and EMA (Appendix 1: Acceptable intakes established for N-nitrosamines), the acceptable daily intake for N-Nitroso-duloxetine is 100ng, which translates to a specification limit of around 0.8ppm in the drug product, considering a maximum daily dose of 120mg for Duloxetine.
Duloxetine is indicated for the treatment of Major Depressive Disorder (MDD), Generalized Anxiety Disorder (GAD), and neuropathic pain.
Nitrosamine Risk in Duloxetine
Duloxetine is prone to nitrosamine impurities risk, with around 13 recalls reported for Duloxetine for N-Nitroso Duloxetine between 2023 and 2024 from different manufacturers (Towa Pharmaceutical– 7 recalls, Aurobindo – 6 recalls). Duloxetine is a secondary amine molecule with five alpha hydrogen atoms around the amine functional group, making it prone to forming the nitrosamine impurity, N-Nitroso duloxetine, when facilitating conditions such as the presence of nitrites and acidic conditions are present.
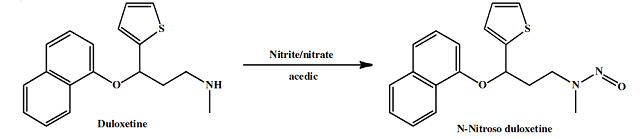
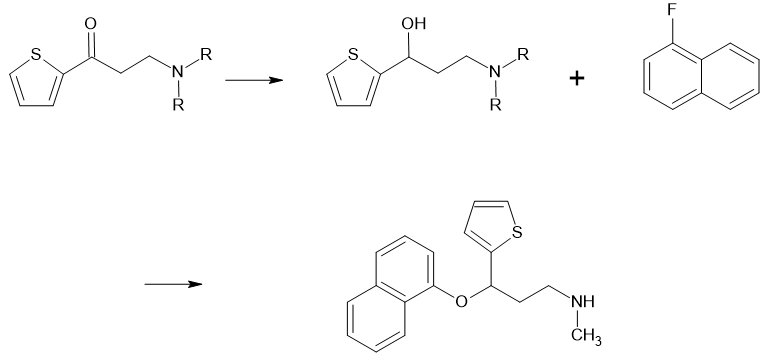
However, the general synthetic pathway for Duloxetine does not involve any nitrosating reagents. The typical synthesis route involves starting with a keto compound, typically dimethylamino-1-(2-thienyl)-1-propanone, which is reduced to a hydroxy compound and then condensed with 1-fluoronaphthalene. There are no nitrosating reagents in the API synthesis route, so the likelihood of forming the NDSRI during API synthesis is very low.
Presence of Nitrites in Excipients
The presence of nitrites in excipients like sucrose and talc used in the manufacture of the drug product Duloxetine can cause the formation of the NDSRI. For molecules like Duloxetine, which have a higher propensity for forming NDSRI impurities, controlling nitrites in excipients used in the manufacture of the drug product becomes critical. A database of nitrite concentrations in common excipients maintained by Lhasa Limited could be a useful tool for selecting sources of excipients with low nitrite levels and reducing the risk of nitrosamine formation.
References:

For More On Nitrosamines and NDSRIs
Leave a Comment
You must be logged in to post a comment.