FDA 483 to Catalent Indiana Flags Inadeq
Novo Nordisk owned Catalent Indiana sterile products formulation facility was inspected by USFDA in June

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Novo Nordisk owned Catalent Indiana sterile products formulation facility was inspected by USFDA in June
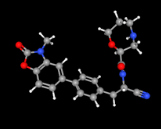
USFDA has approved Insmed’s Brinsupri (Brensocatib) for Bronchiectasis, a chronic drug disease. The medicinal product

In a blog posted on 8 August 2025, MHRA’s Chris Jones (IAG Chair) and Himal Makwana

The European Commission (EC) has published a draft guidance – Annexure 22 – Artificial Intelligence

USFDA has issued a Warning letter to Glenmark Pharma’s Pithampur manufacturing facility (FEI 3008565058). The

China’s rise in cutting-edge pharmaceutical innovation received a major fillip with the USFDA approval of

USFDA has published over 200+ redacted Complete Response Letters (CRLs) issued to drug applicants. The
USFDA has allowed drug manufacturers additional time for submission of mitigation actions and required changes

Indian drug regulator CDSCO has published the Not of Standard Quality (NSQ) alert lists for

USFDA has issued the final guidance for Pre-Submission Facility Correspondence (PFC) for prioritised generic drug