FDA approves NCE Gepirone (EXXUA) for Ma
Fabre-Kramer Pharmaceuticals Inc. (Fabre-Kramer), a biopharmaceutical company announced approval of Exxua™ (gepirone hydrochloride extended-release tablets)

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Fabre-Kramer Pharmaceuticals Inc. (Fabre-Kramer), a biopharmaceutical company announced approval of Exxua™ (gepirone hydrochloride extended-release tablets)

Florida based VistaPharm LLC is recalling in US one lot of ulcer treatment drug Sucralfate

In the guidance FDA describes use of alternative tools to assess manufacturing facilities identified in

FDA has raised concerns about therapeutic equivalence of Tacrolimus capsules of Accord Healthcare to brand

Sandimmune® Oral Solution (cyclosporine oral solution, USP), 100 mg/mL is indicated for the prophylaxis of

Abbot recalls all batches of Digene Gel of all flavours (Mint, Orange, Mix fruit flavours)
USFDA has eliminated the REMS for Lotronex (alosetron hydrochloride) and approved generics. Alosetron hydrochloride is

USFDA has announced funding opportunity for FDA Orphan Products Clinical Trials Grant Program. The deadline

Bottles of Digoxin Tablets, USP 0.125mg are incorrectly labelled and contain Digoxin Tablets USP, 0.25mg
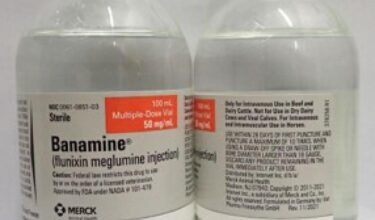
Flunixin meglumine is a potent, non-narcotic, nonsteroidal, analgesic agent with anti-inflammatory and antipyretic activity. It