FDA Issues guidance for Handling and Ret
FDA has issued a new guidance for handling reserve samples from bioavailability (BA) and bioequivalence

Warning letters, 483s, Recalls, Import Alerts, Audit observations

FDA has issued a new guidance for handling reserve samples from bioavailability (BA) and bioequivalence

USFDA has approved Italfarmaco’s Duvyzat (givinostat) oral medication for treatment of Duchenne Muscular Dystrophy (DMD).

USFDA has approved Idorsia’s new drug Tryvio, a New Molecular Entity (NME) drug Aprocitentan for
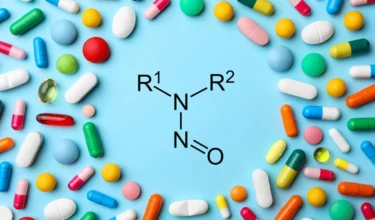
EMA(Europeans Medicines Agency) has updated the Appendix 1 Acceptable intakes established for N-nitrosamines with 15

USFDA has updated Safety Communication on Chinese made plastic syringes. FDA had issued a Safety

USFDA had inspected Aurobindo’s Eugia SEZ Pvt Ltd facility at Polepally, Telengana in India in

USFDA has issued a new guidance for primary batches to be included in the CMC
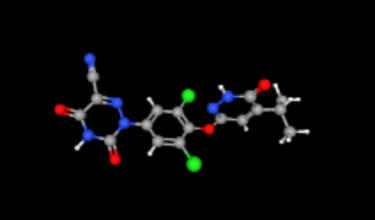
FDA has approved Madrigal Pharmaceuticals’ Rezdiffra (Resmetirom) for treatment of fatty liver disease (Noncirrhotic Nonalcoholic

Dublin based Par Pharmaceutical, part of Endo International plc is recalling one lot of Treprostinil

Eugia US LLC is recalling three lots of Methylprednisolone acetate Injectable Suspension (80mg/mL, 5mL Multiple-Dose