Dr.Reddy’s Recall Several Lots of Ibup
Dr.Reddy’s laboratories Inc (DRL) has initiated recall of 50 batches of Ibuprofen tablets in US

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Dr.Reddy’s laboratories Inc (DRL) has initiated recall of 50 batches of Ibuprofen tablets in US

Zydus Pharmaceuticals (USA) Inc initiated recall of multiple lots of Verapamil Hydrochloride injection due to

Glenmark Pharmaceuticals is recalling 114 batches of Potassium Chloride Extended-Release Capsules, USP (750 mg) 10

Eugia US LLC is recalling two more injectable products in US for failed impurities, degradation
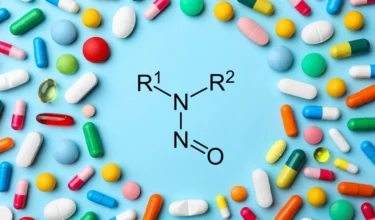
Glenmark recalled several lots of Rizatriptan Benzoate Tablets and Rizatriptan Benzoate Orally Disintegrating Tablets in

Dr.Reddy’s (DRL) is recalling 1176 bottles of Sirolimus 1mg tablets (NDC 55111-653-01) in US for

WHO has issued an alert on falsified Propylene glycol bearing name of Dow detected in

J&Js paediatric cough syrup Benylin is being recalled in several African countries following detection of

Natco Pharma is recalling one more lot of Lanzoprazole 15 mg delayed release capsules for

Glenmark has initiated a recall of one lot of Diltiazem Hydrochloride 120mg, Extended Release(ER) Capsules