New NSCLC Drug Approved by USFDA –
China’s rise in cutting-edge pharmaceutical innovation received a major fillip with the USFDA approval of

Warning letters, 483s, Recalls, Import Alerts, Audit observations

China’s rise in cutting-edge pharmaceutical innovation received a major fillip with the USFDA approval of

The U.S. Food and Drug Administration (FDA) has approved Sanofi’s Qfitlia (fitusiran) for the treatment
USFDA has approved Checkpoint Therapeutics Cosibelimab-ipdl (Unloxcyt) for treatment of skin cancer CSCC (metastatic cutaneous squamous

The USFDA has approved Neurocrine Biosciences’ new drug, Crenessity (Crinecerfont), for the treatment of classic
USFDA has approved Inavolisib ((Itovebi) of Genentech in combination with palbociclib and fulvestrant, for the

FDA has given approval for Janssen’s New Molecular Entity Lazertinib (Lazcluze). Approval is accorded for

Gilead announced FDAs approval of Livdelzi (Seladelpar) for treatment of PBC, a rare, chronic, autoimmune

FDA approved Akebia’s Vafseo (Vadadustat) for treatment of anaemia due to Chronic Kidney Disease (CKD)
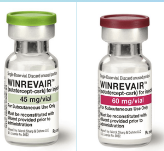
FDA has approved Merck’s Biologic drug Winrevair (Sotatercept-CSRK) for Pulmonary Arterial Hypertension (PAH). The product

USFDA has approved Italfarmaco’s Duvyzat (givinostat) oral medication for treatment of Duchenne Muscular Dystrophy (DMD).