Nitrosamine Impurities in Pharmaceutical
Qvents Apps & ToolsNitrosamine and NDSRI Risk Assessment Tools – APIs, Drug Products, Excipients, Water

Warning letters, 483s, Recalls, Import Alerts, Audit observations
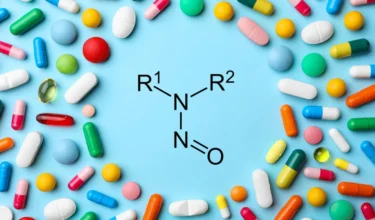
Qvents Apps & ToolsNitrosamine and NDSRI Risk Assessment Tools – APIs, Drug Products, Excipients, Water
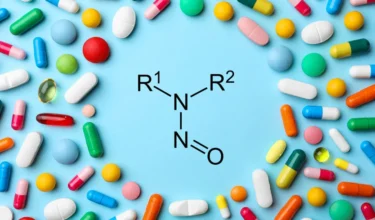
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Adding to the number of recalls during 2024 for NDSRIs, Ascend (Alkem) is recalling the
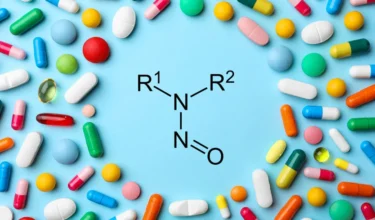
Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has