NDSRI Nitrosocinacalcet: Aurobindo Recal
Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has

Warning letters, 483s, Recalls, Import Alerts, Audit observations
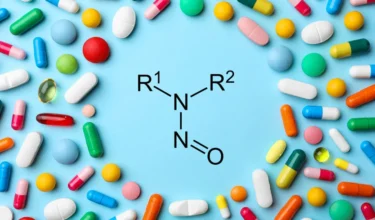
Aurobindo is recalling Cinacalcet tablets in US due to nitrosamine NDSRI impurity N-Nitroso-cinacalcet. Aurobindo has

Authored by: Venkiteswaran.T.K, SubrangshuChourdhary, Srinivas Churya, Satish Reddy, Sanjeev Kumar Singh Aarti Drugs API facility in Tarapur, India

Authored by: Venkiteswaran.T.K, Subrangshu Chourdhary, Satish Reddy Lupin is recalling over 600,000 bottles of Ramipril capsules across different

Authored by: Venkiteswaran.T.K, Subrangshu Chourdhary, Srinivas Churya Jubilant Generics Roorkee facility was inspected in January

USFDA inspected Granules India’s Finished Dosage and PFI facility (FEI 3004097901) at Gagillapur Village, Hyderabad

In October 2024, Accord Healthcare recalled over 25 lots of Cinacalcet tablets in various strengths

With contributions from: Venkiteswaran.T.K; Satish Reddy; Shashank Sharma; Srinivas Churya; Subrangshu Choudhary The USFDA inspection

With contributions from: Venkiteswaran.T.K; Satish Reddy; Shashank Sharma; Srinivas Churya; Subrangshu Choudhary, Veena Raj The USFDA Form

Sun Pharma’s Dadra Unit was issued FDA Warning letter in June 2024, following critical observations

Laurus Synthesis was issued an Untitled Letter by USFDA in May 2024, following critical observations