Metallic Particles in APIs, Complaint In
The USFDA issued a Warning letter to Hikal’s API facility in Jigani, Bangalore, India. The

Warning letters, 483s, Recalls, Import Alerts, Audit observations
The USFDA issued a Warning letter to Hikal’s API facility in Jigani, Bangalore, India. The
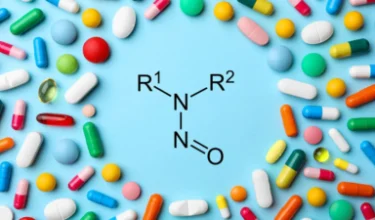
Alembic recently initiated recall of antidepressant drug Doxepin Hydrochloride Capsules (10 mg) in US for Nitrosamine

The USFDA has issued a Warning Letter to Shiva Analyticals’ Bangalore facility following significant cGMP

USFDA issued a Warning letter to Glenmark Facility at Pithampur (FEI 3008565058) in July 2025.

Natco Pharma’s sterile products manufacturing facility at Rangareddy, Telengana (FEI 3004540906) was inspected by USFDA

First Recall of Diphenhydramine HCl for NDSRI With timelines closing in for drug product manufacturers

Piramal Pharma’s API unit at Navi Mumbai (FEI 3008763768) was issued USFDA Form 483 with

The FDA inspected Colgate-Palmolive/Tom’s of Maine, Inc’s Sanford facility in the US in May 2024.
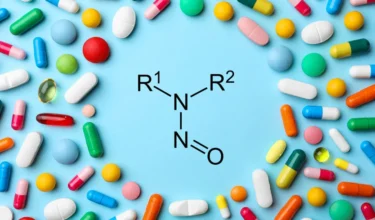
Glenmark Pharmaceuticals is recalling several lots of the cardiovascular drug Carvedilol in US due to

Adding to the number of recalls during 2024 for NDSRIs, Ascend (Alkem) is recalling the