

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations
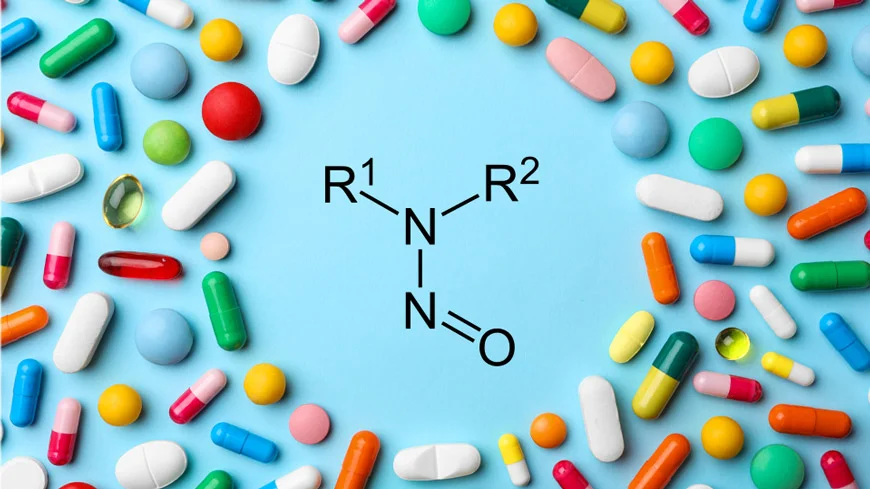
USFDA has allowed drug manufacturers additional time for submission of mitigation actions and required changes for nitrosamine impurities as recommended under RAIL guidance.
In August 2023, FDA issued the RAIL guidance (Recommended Acceptable Intake Limits for Nitrosamine Drug Substance-Related Impurities (NDSRIs) with recommended acceptable intake limits for NDSRIs. FDA’s Nitrosamine Guidance introduced and elaborated a three-step mitigation strategy for nitrosamines in drug products
The RAIL guidance recommended that confirmatory testing for drug products at risk of NDSRI contamination and submission of related changes be completed by August 1, 2025. While the FDA continues to emphasize this target date, it is now allowing additional time for applicants to submit the required changes. The update is published on the FDAs webpage CDER Nitrosamine Impurity Acceptable Intake Limits
Applicants not able to submit the necessary changes by the timeline should provide a progress report by 1 August 2025 which should include:
This information should be included in the Annual Report (AR) where the AR due by August 1, 2025. If the AR for this cycle has already been submitted, applicants should update the Log of Outstanding Regulatory Business section in the AR to include the information on the FDA’s nitrosamine-related recommendations.
Manufacturers of non-application products (e.g., OTCs), who typically do not submit annual reports, are also expected to prepare a progress update and retain documentation for FDA review upon request.
References:
Leave a Comment
You must be logged in to post a comment.