

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations
Zydus is recalling multiple lots of the antipsychotic drug Chlorpromazine in U.S due to the presence of the impurity N-nitroso-desmethyl-chlorpromazine (NDSRI) at levels exceeding the recommended acceptable intake (AI) limit. The recall affects tablets in a range of strengths: 10 mg, 25 mg, 50 mg, 100 mg, and 200 mg. The NDSRI N-nitroso-desmethyl-chlorpromazine has a recommended AI limit of 26.5 ng/day based on the predicted Carcinogenic Potency Categorization Approach (CPCA) as per USFDA CDER Nitrosamine Impurity Acceptable Intake Limits.
There are several generic version of Chlorpromazine in US including from Dr.Reddy’s, Eugia, Alembic, Aurobindo, Lupin, Teva, Glenmark and others. There was a similar recall of Chlorpromazine tablets by Glenmark earlier in the year for NDSRI.
Potential Formation of NDSRI in Chlorpromazine
Chlorpromazine has a tertiary amine functional group in the structure. Theoretically tertiary amines have lower propensity to formation of nitrosamine impurities compared to secondary amines, still risk of formation of nitrosamine impurities through a N-Nitroso-dealkylation exist.
The NDSRI can form during the manufacturing or storage of the drug product. The most likely cause is the interaction of trace levels of nitrites (typically from excipients) with the tertiary amine group. The nitrites react with the tertiary amine, to form a secondary amine intermediate by dealkylation. This intermediate then reacts further with nitrites to form the nitrosamine impurity.
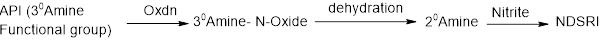
The active pharmaceutical ingredient (API) synthesis for chlorpromazine typically involves a reaction between 2-chlorophenothiazine and 3-dimethylaminopropyl chloride. This process does not use nitrosating agents, so the risk of NDSRI formation from the API itself is considered very low.
However, common excipients like Microcrystalline Cellulose, Lactose Monohydrate, Hypromellose, Magnesium Stearate, Povidone etc. which is often involved in the Chlorpromazine tablet formulations can contain trace levels of nitrites. Under favourable conditions, the nitrites can react with the tertiary amine group of chlorpromazine leading to the formation of the NDSRI.
Key Takeaways:
Regulatory agencies require drug manufacturers and marketing authorisation applicants to have a robust risk assessment and a control strategy in place to minimize the potential for nitrosamine impurities in the drug products.
As seen in most NDSRI cases reported, controlling nitrosating impurities in excipients is crucial for mitigating the risk of NDSRI formation in drug products which has vulnerable actives (APIs) with secondary or tertiary amine functional group in the structure. This calls for a thorough risk assessment of all input materials for nitrosating impurities, identification of al potential sources for formation of nitrosamines and NDSRIs in the product and implementation of a control strategy. The control strategies could range from:
References:
Leave a Comment
You must be logged in to post a comment.