

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations



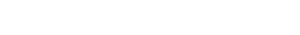

USFDA 483 / UCB Farchim SA/ June 2023 / Deficiencies in Method Transfer, Material receipt
USFDA Inspected UCB Farchim site at Bulle, Switzerland (FEI 3005023799) in June / July 2023. The site was inspected by USFDA investigator Arsen Karapetyan and was issued Form 483 with four observations. These include gaps in method verification / validation programs and inadequate procedures for material receipt and handling. The Form 483 also cited fundamental cGMP deficiencies in Data Integrity programme and electronic data review and controls.
Firm has not performed analytical method transfers for (specific) active pharmaceutical Ingredients. Standard and sample solution stability has not been established as part of the HPLC method validation reports
Written procedures for Warehouse facility operations lack sufficient details for material receipt activities.
The USFDA (CFR 211.165, 211.194) require that accuracy, sensitivity, specificity, and reproducibility of test methods employed … shall be established and documented. The suitability of all testing methods used shall be verified under actual conditions of use. ICH Q2 (R1) – Validation of Analytical Procedure guideline recommends that If measurements are susceptible to variations in analytical conditions – example stability of analytical solutions – the analytical conditions should be suitably controlled, or a precautionary statement should be included in the procedure (8. Robustness). USP 1224 – Transfer of Analytical procedures define method transfer as an activity that qualifies a laboratory to use a method originated in another laboratory.
<Guidelines on Method Validations>
It is imperative that analytical methods are either fully validated or methods are verified (if it’s a compendial method) or formally transferred from the originating laboratory to the receiving laboratory to comply to the requirement that the method is verified under actual conditions of use.
If a drug product manufacturer adopts a method for raw material (API) from the supplier and its fully validated at the supplier end, still a method transfer (can be achieved by a revalidation or partial validation) should be performed and documented. This shall be defined in the Firm’s Validation Policy and Method Validation procedures. The robustness studies conducted as part of method validation must verify the stability of the solution (to ascertain susceptibility of the method to variations in analytical conditions – like time delay for injection of standard / sample solutions). Based on the studies solution stability time must be defined in the analytical procedure.
Procedures for material receipt in warehouse must be described and ensured through appropriate checklists covering visual examination, labelling, name of material, supplier name & details, whether it an approved supplier, container damage, tampering of seals, contamination and any other critical factor. Deviations should be documented, assessed before material is accepted.
<Refer GMP Guidelines and requirements: USFDA CFR 211.80, 211.82, ICH Q7A: Section 7.2>
Leave a Comment
You must be logged in to post a comment.