FDA clears Bovaer – Elanco’s Met
Elanco Animal Health announced that the USFDA has completed its comprehensive, multi-year review of Bovaer

Warning letters, 483s, Recalls, Import Alerts, Audit observations
Elanco Animal Health announced that the USFDA has completed its comprehensive, multi-year review of Bovaer

FDA approved Akebia’s Vafseo (Vadadustat) for treatment of anaemia due to Chronic Kidney Disease (CKD)
FDA has approved Merck’s Biologic drug Winrevair (Sotatercept-CSRK) for Pulmonary Arterial Hypertension (PAH). The product

USFDA has approved Italfarmaco’s Duvyzat (givinostat) oral medication for treatment of Duchenne Muscular Dystrophy (DMD).

USFDA has approved Idorsia’s new drug Tryvio, a New Molecular Entity (NME) drug Aprocitentan for
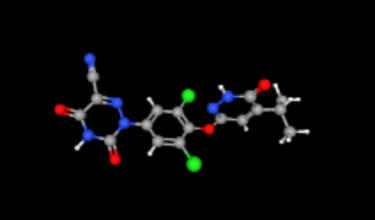
FDA has approved Madrigal Pharmaceuticals’ Rezdiffra (Resmetirom) for treatment of fatty liver disease (Noncirrhotic Nonalcoholic
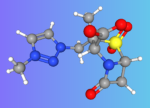
Indian Pharma company Orchid Pharma’s New Molecular Entity (NME) Enmetazobactam gets USFDA approval for treatment

FDA has approved Novartis’ Iptacopan (Fabhalta) for treatment of rare blood disease, Paroxysmal Nocturnal Hemoglobinuria

Desmoid tumors are non-cancerous, but locally aggressive and invasive soft-tissue tumors that can lead to

AstraZeneca’s TRUQAP (New Molecule Capivasertib) in combination with Faslodex (Fulvestrant) has been approved in the