
Qvents Apps & Tools
Check out the Excipient Nitrosamine Risk Assessment Tool!! Fill in the Questionnaire responses, Get the Nitrosamine risk and Carryover Potential for Nitrites, Secondary / Tertiary amines – Click Here (For more Apps & Tools visit Tejoh.com)
Authored by: Venkiteswaran.T.K
Nitrosamine impurities in pharmaceutical products are a significant concern due to their potential carcinogenicity and the strict regulatory requirements for their control. Regulatory agencies and Technical Forums including USFDA, EMA, WHO, PIC/S, ICH, and others, mandate robust risk assessment and control strategy to minimize nitrosamine contamination of pharmaceutical products and ensure patient safety. This document outlines the contributing factors for nitrosamine formation and provides a structured approach to their management, supported by Qvents’ comprehensive templates for risk assessment and supplier qualification. These templates enable systematic evaluation of nitrosamine risks across APIs, excipients, process water, and drug product manufacturing processes, facilitating the development of effective mitigation measures.
Sources of Nitrosamine Formation
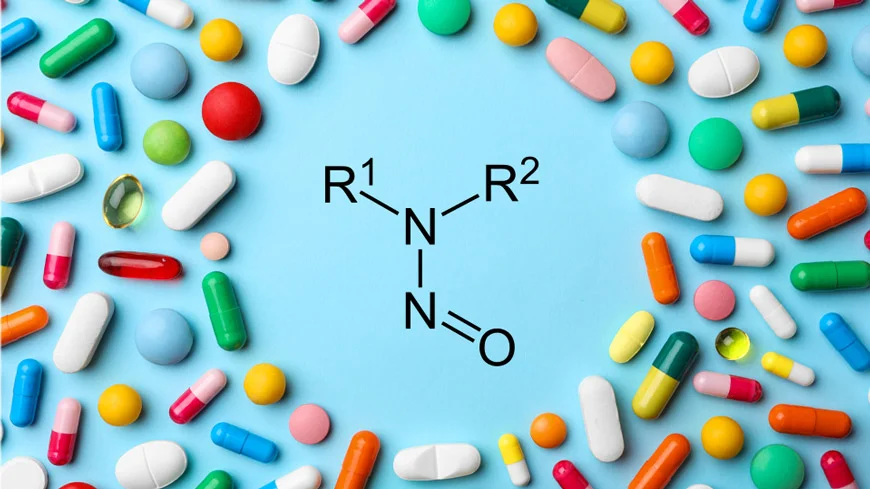
Nitrosamines are typically formed through nitrosation reactions, which involve amines (especially secondary and tertiary amines) reacting with nitrous acid. Nitrous acid is generated under acidic conditions from nitrite salts or other nitrosating agents.
However a range of nitrosating factors can potentially cause generation of nitrosamine impurities in Drug products, APIs, Excipients, Process Water
- Nitrosating agents: Nitrites (e.g., sodium nitrite, NaNO2) and nitrous acid (HNO2), nitric oxide (NO), nitric oxide due to nitric acid, nitrosyl halides (e.g., ClNO, BrNO), dinitrogen trioxide (N2O3), dinitrogen tetroxide (N2O4) and organic nitrites (e.g., t-BuONO), Ozone with amines leading to nitrites, Chloramine
- Secondary and tertiary amines
- Other sources of secondary amines: Amide based solvents such as N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMAC) and N-methyl pyrrolidinone (NMP), quaternary ammonium salts such as tetrabutylammonium bromide (TBAB), tertiary amine bases, primary amines such as methylamine, azides. Presence of such reagents along with nitrosating agents in the process lead to moderate risk of nitrosamine
Specific types of nitrosamines, known as Nitrosamine Drug Substance Related Impurities (NDSRIs), result from the nitrosation of amine functional groups in APIs, API degradants, intermediates, or impurities. These impurities may form during:
- API manufacture.
- Formulation manufacturing.
- Storage of API or formulation products.
In addition to formation of nitrosamines during chemical synthesis of APIs due to interaction of amine residues with nitrosating groups present in the synthesis, nitrosamine can stem from external sources, including:
- Excipients containing amine residues or nitrosating agents.
- Water treatment procedures, such as chloramination, ozonation, or leaching of amine residues from quaternary ammonium salts in ion exchange resins.
- Shared equipment with processes involving nitrosating factors or contamination from recovered solvents. (Even if an API or excipient process itself do not have the nitrosating factors, sharing of equipment with another product which has the nitrosating factors can cause contamination).
- Packaging materials, such as printed lidding foils containing nitrocellulose in adhesives and amine residues in inks.
Risk Assessment and Control Strategy
The wide array of potential contributors to nitrosamine formation necessitates a robust risk assessment and control strategy, as emphasized by USFDA and EMA guidance on nitrosamines. Effective control strategies should include:
- Supplier Qualification Programs for APIs and excipients to identify and minimize risks of nitrosamine impurities and nitrosating factors.
- Monitoring of APIs, excipients, and water for nitrosamines, nitrites, and amines, with appropriate control measures where required.
- Stability Studies to monitor nitrosamines and NDSRIs over the shelf life of materials, especially when contributing factors are present in the product. (Even if nitrosamines are not present at the time of batch release, presence of amine residues, amine functional groups, generation of amine functional groups due to degradation and nitrosating agents are contributing factors for formation of nitrosamines during shelflife)
- Implementation of preventive measures in drug product manufacturing, such as:
- Use of nitrite scavengers (e.g., ascorbic acid).
- Replacement of excipients with higher risk of nitrosating factors or selection of alternate sources, reformulation of the product.
- Specification control for nitrosamine or nitrosating factors in APIs, excipients, and water
- Evaluation of Packaging Materials, focusing on nitrosating precursors like nitrocellulose and amine residues.
- Advanced Analytical Methods for detecting trace levels of nitrosamines and structurally diverse NDSRIs (e.g., LC-MS/MS, GC-MS).
Templates for Risk Assessment
Qvents presents a comprehensive set of templates for supplier qualification, process evaluation, and risk assessment of APIs, excipients, process water, and drug product manufacturing. These templates are designed to guide systematic identification and mitigation of nitrosamine risks:
- Supplier Assessment and Risk Evaluation Templates for APIs.
- Supplier Assessment and Risk Evaluation Templates for Excipients.
- Risk Assessment Template for Process Water, considering nitrosamine generation during treatment methods.
- Nitrosamines and NDSRIs Risk Assessment Template for Drug Products, drawing from evaluations of APIs, excipients, and process water.
Implementation Strategy
Use these templates to develop your own formats with applicable factors and questionnaires for risk evaluation. Based on the results, actions to mitigate nitrosamine risks may include:
- Specification controls for nitrosamine levels in product components.
- Regular monitoring of APIs, excipients, and water for contributing nitrosation factors.
- Replacement of excipients or changing sources with higher risks.
- Process water controls to mitigate nitrosating impurity risks.
- Reformulation of drug products, including introducing nitrogen scavengers.
- Stability monitoring during product shelf life for nitrosamines or NDSRIs.
Templates:
References:





Leave a Comment
You must be logged in to post a comment.