

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations
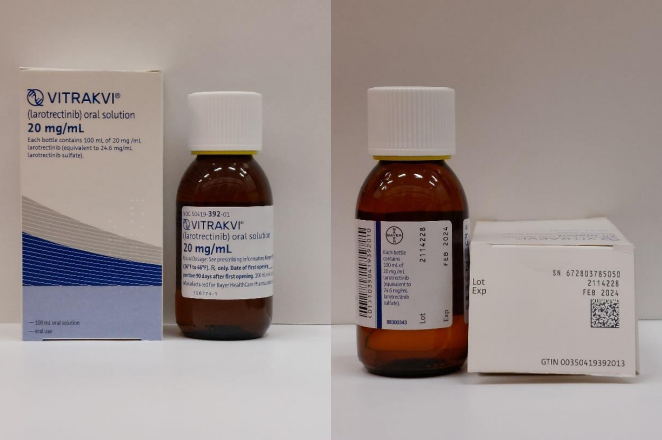
Bayer is recalling one lot of Vitrakvi® (larotrectinib) Oral Solution 20 mg/mL in 100mL glass bottles due to microbial contamination identified as Penicillium brevicompactum observed during routine ongoing stability testing. Vitrakvi is indicated for the treatment of solid tumors and patients on Vitrakvi® may be immunocompromised. There is a probability that ingestion of Penicillium brevicompactum in patients may result in invasive fungal infections of the blood or pneumonia that can be life-threatening.
Leave a Comment
You must be logged in to post a comment.