FDA issues draft guidance for Dietary In
The USFDA released draft guidance on New Dietary Ingredient Notification (NDIN) Master Files for dietary

Warning letters, 483s, Recalls, Import Alerts, Audit observations

The USFDA released draft guidance on New Dietary Ingredient Notification (NDIN) Master Files for dietary

FDA has issued a new guidance for handling reserve samples from bioavailability (BA) and bioequivalence
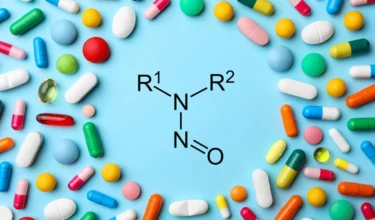
EMA(Europeans Medicines Agency) has updated the Appendix 1 Acceptable intakes established for N-nitrosamines with 15

USFDA has issued a new guidance for primary batches to be included in the CMC

European Pharmacopoeia Commission (EPC) approved the new strategy for N-nitrosamine impurities in individual monographs in

Each Drug Establishments (Registrants*) registered with FDA must provide information on amount of each listed

The draft guidance Good Manufacturing Practice for Active Pharmaceutical Ingredients Used in Veterinary Medicinal Products

This guidance provides procedures for ANDA applicants who wish to pursue a request for reconsideration

FDA has published a revised guidance on quality considerations for topical ophthalmic drug products –

The revised monograph for Propylene Glycol (PG) includes test for Ethylene Glycol (EG) and Diethylene