Lupin USFDA 483 Cites Lapses in OOS Inve
USFDA 483 to Lupin Pithampur in Mar 2023 (Inspectors Eileen A Liu, Yvins Dezan) cites deficiencies

Warning letters, 483s, Recalls, Import Alerts, Audit observations

USFDA 483 to Lupin Pithampur in Mar 2023 (Inspectors Eileen A Liu, Yvins Dezan) cites deficiencies

USFDA inspection of Cipla Pithampur site ( FEI 3008581988) by Inspectors Saleem A Akhtar &

USFDA Inspection of Intas, Matoda, Sanand facility in India (FEI 3004011473); from 22 November 2022 to

Macleods recalled 10052 bottles of Levofloxacin USP 500mg tablets in January 2023 due to mismatching

Warning letter / Glenmark, Goa, India /FEI 3004672766 / MARCS-CMS 637314/ 320-23-04/ NOVEMBER 22, 2022/
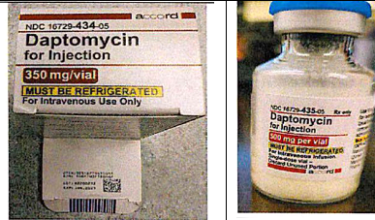
Accord healthcare recalled from US market Daptomycin for Injection 500 mg/vial and Daptomycin for Injection 350 mg/vial

USFDA Warning letter to Dupont cites inadequate evaluation of complaints, even though the Firm received several

USFDA Warning letter to Dupont cites inadequate OOS investigations, Root cause and CAPA, Impact evaluation. The warning letter

USFDA Warning letter to Dupont cites failure to ensure test methods are suitable, use of Non

USFDA Warning letter to Dupont cites inadequate change management program. The warning letter to Dupont Nutrition USA in