Imprimis Recall Unapproved Ophthalmic In
Imprimis NJOF recalled three intraocular ophthalmic injection drug products in December 2025 (as per USFDA

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Imprimis NJOF recalled three intraocular ophthalmic injection drug products in December 2025 (as per USFDA

Qvents provides useful tools for QMS processes – Risk Assessments, Continuous Process Validations, Supply Chain Traceability
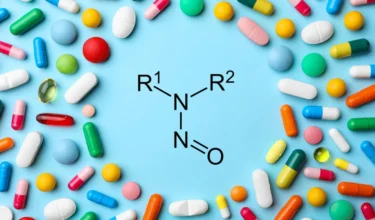
Qvents Apps & ToolsNitrosamine and NDSRI Risk Assessment Tools – APIs, Drug Products, Excipients, Water
As per EUGMP requirements, EudraLex, Annex 16: Certification by a Qualified Person and Batch Release,
Author: Venkiteswaran.T.K The USFDA’s 2011 guidance on process validation advocates a life cycle approach and

Qvents Apps & Tools Access the Excipient Risk Assessment Tool – Click Here According to

Authored by: Srinivas Churya This Quality Insight post presents a concise approach for selection and

The FDA inspected Colgate-Palmolive/Tom’s of Maine, Inc’s Sanford facility in the US in May 2024.

Authored by: Srinivas Churya In the pharmaceutical industry, analytical division and analytical laboratories plays a

Five different facilities of Eugia across India and US were inspected by USFDA between December