Unichem Recalls Hypertension Tablets for
Unichem Pharmaceuticals (USA), Inc. initiated a recall of Bisoprolol fumarate and Hydrochlorothiazide tablets in the

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Unichem Pharmaceuticals (USA), Inc. initiated a recall of Bisoprolol fumarate and Hydrochlorothiazide tablets in the

USFDA Inspection of Pharmathen’s Rodopi facility in Greece (FEI 3009961173) resulted in issuance of Form

Imprimis NJOF recalled three intraocular ophthalmic injection drug products in December 2025 (as per USFDA

Qvents provides useful tools for QMS processes – Risk Assessments, Continuous Process Validations, Supply Chain Traceability

In November 2025, the USFDA issued a Warning Letter to Bangalore-based Cdymax India following an

The USFDA inspection of Hetero Labs Ltd, Unit-IX (FEI: 3009093782), Narasapuram, India, conducted in September
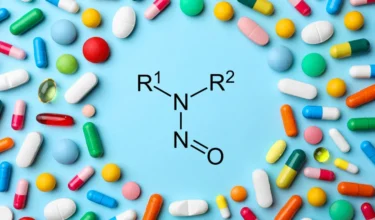
Zydus is recalling multiple lots of the antipsychotic drug Chlorpromazine in U.S due to the

Accord (Intas) initiated a recall of several lots of Levothyroxine tablets in the US for
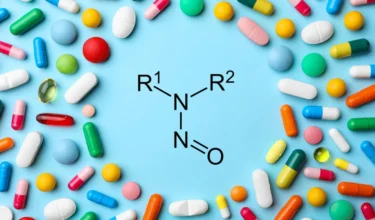
Qvents Apps & ToolsNitrosamine and NDSRI Risk Assessment Tools – APIs, Drug Products, Excipients, Water
As per EUGMP requirements, EudraLex, Annex 16: Certification by a Qualified Person and Batch Release,