

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations



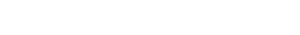

Warning letter / Centrient India / MARCS-CMS 640196/ 320-23-06/ DECEMBER 07, 2022/ Observation 3
The USFDA issued a Warning Letter to Centrient India’s Toansa, Punjab facility (FEI 3004497364) in December 2022, following an inspection conducted from June 23 to July 1, 2022 by investigators Marcellinus D. Dordunoo and Joel D. Hustedt. One of the critical observations cited lapses in investigating black particles in API batches, inadequate root cause analysis, and ineffective CAPAs.
Failure to establish and follow written procedures for investigating critical deviations or the failure of API batches to meet specifications.
Failed to adequately investigate and determine the root cause of black particles in two batches of API. Investigation report stated the black particles were non-metallic charred product residue, but failed to provide data to support the conclusion.
Firms response indicated installation of (system) to limit presence of metal particles. However, investigation remains inadequate because, firm did not provide the data to support proposed root cause or identify an adequate CAPA. The CAPA does not address non-metallic sources of contamination such as charred product residue or inadequate cleaning or fully address metallic sources of contamination such as reactive, additive, or absorptive product contact surfaces.
Firm to provide:
A comprehensive assessment of overall system for investigating deviations, discrepancies, complaints, out-of-specification (OOS) results, and failures. To provide a detailed action plan to remediate this system – significant improvements in investigation competencies, scope determination, root cause evaluation, CAPA effectiveness, quality unit oversight, and written procedures. Address how your firm will ensure all phases of investigations are appropriately conducted.
An investigation into the observation of black particles in API batches concluded the particles are non metallic, dissolves in solution and is charred product residue, but failed to provide data to support the conclusion. Yet in the response provided to FDA, Firm indicated it has installed (system) to limit presence of metal particles. FDA commented that the investigations into the incident are inadequate, does not support root cause and fail to identify adequate CAPA. The CAPA does not does not address non-metallic sources of contamination such as charred product residue or inadequate cleaning or fully address metallic sources of contamination such as reactive, additive, or absorptive product contact surfaces
FDA directed the firm to perform a comprehensive assessment of its systems for investigating deviations, discrepancies, complaints, OOS results, and other failures, along with a detailed remediation plan.
Investigation of Quality events, identification of root cause and CAPA and documentation should be sound and comprehensive. The investigation conclusions need to be objective.
For example, in the cited case, is it metallic particle, charred product residue or could it be either of the two. Problem definition, investigation, rationale for root cause(s) identified and actions taken should be consistent. All factors that can contribute to the issue should be evaluated for arriving at the root cause or probable root causes. Reasons for zeroing in on the root cause and also rejecting some of the probable causes considered should be documented with sound rationale. If a clear root cause is not identified, and only probable causes are identified, CAPAs should be implemented for each.
The CAPA should address the root cause and root cause should be an outcome of investigation.
For example, a system to prevent metallic particle contamination may be good to have, but when the investigation identifies the particles as non-metallic, it does not address the root cause and will not prevent recurrence of the issue.
The investigation conclusions should be justified and scientific.
A comprehensive investigation with appropriate investigation tools helps identify probable root causes. For example:
CAPA Effectiveness
After implementation of the CAPA, the effectiveness of each implemented CAPA should be reviewed in a reasonable timeframe. An effective CAPA will mean the problem or deviation is not recurring.
Quality and Operations teams should be trained in investigation methodologies. Case studies and workshops strengthen skills in root cause analysis and CAPA design.
When a Firm is hit with such observations of Inadequate Investigation-Root cause-CAPA in a regulatory inspection (or even other audits), the response should be thorough and well structured. The response should address not only the cited deficiencies, but also the evaluate, extent of such discrepancies and why the investigations were deficient in the first place.
The Warning letter highlights that Investigation procedures are deficient. This implies there could be such lapses in other failure investigations as well. This means a review all discrepancies logged in the past – deviations, OOS, complaints, other failures – to evaluate whether each investigation was thorough, root causes were identified and appropriate CAPAs were implemented. Perform an objective review and assessment against a checklist (but not limited to) – problem definition, is root cause(s) identified and scientific, does CAPA address the root cause, is CAPA effective – are there repeat incidents after implementation of CAPA. The scope of such review should cover a sufficient number of past incidents for an effective assessment. For example, a time frame covering all distributed batches within valid expiry or all failure investigations in the past three years will be justified.
Such a review could result in a list of quality events where investigations were not thorough, root causes were not scientific or CAPAs were inadequate. These events should be reopened for a thorough review to identify all probable root causes and appropriate CAPA.
Review the process and procedures for investigation of discrepancies, investigation tools and understanding and competencies of personnel involved.
The failure investigation procedures should include:
Quality Assurance (QA) should ensure each investigation has followed the process of investigation before accepting / approving the investigation reports and CAPAs.
Quality and management reviews should track trends of discrepancies and failures, recurrence, and CAPA effectiveness.
Teams should undergo competency enhancement programs in investigation techniques and tools, use case studies of past events, other FDA citations.
Failure investigations must be systematic, data-driven, and competency-led. Deficiencies often stem from poor problem definition, superficial evaluation of causes, or irrelevant CAPAs. Such lapses not only invite regulatory action but also risk recurrence of quality failures, undermining product integrity and patient safety.
Leave a Comment
You must be logged in to post a comment.