

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations





Alembic recently initiated recall of antidepressant drug Doxepin Hydrochloride Capsules (10 mg) in US for Nitrosamine Drug Substance Related Impurity (NDSRI) above limits. The USFDA Recommended AI Limits for potential NDSRI impurities in Doxepin Hydrochloride – N-nitroso-desmethyl-cidoxepin and N-nitroso-desmethyl-doxepin, (e)- are 26.5ng per day.
Doxepin Hydrochloride is a tricyclic antidepressant with a tertiary amine (dimethylamino) side chain, which makes it susceptible to nitrosation. Tertiary amines are generally less reactive than secondary amines for nitrosation, but they can still undergo N-nitrosative dealkylation. The nitrosating agent reacts with the tertiary amine functional group cleaving off an alkyl groups to form a secondary amine intermediate which further gets nitrosated. This NDSRI formation can occur in the finished drug product during manufacturing stages or during storage due to trace levels of nitrites from excipients or other sources, especially under facilitating conditions like elevated temperature and humidity.
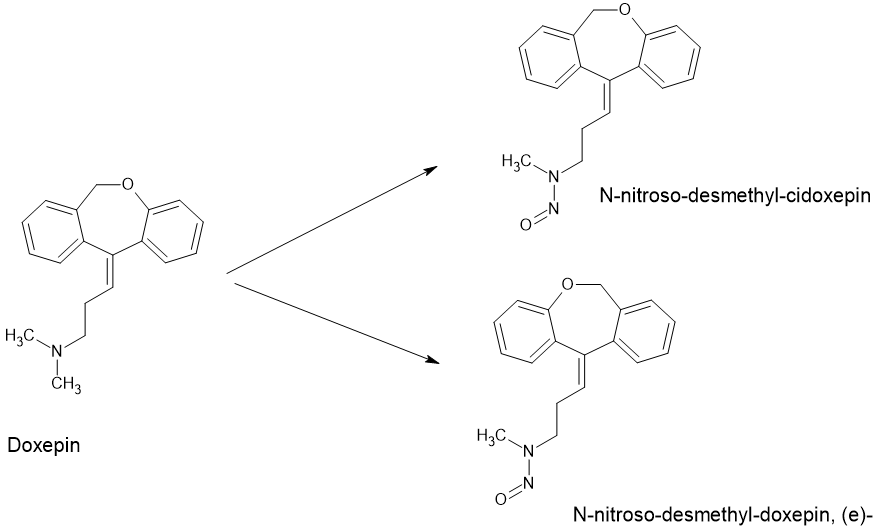
The API synthetic manufacturing process for Doxepin typically involves building the tricyclic core (dibenzoxepinone ring system) from a phthalide intermediate. This is followed by the introduction of the dimethylamino propyl side chain typically by a Grignard reaction with reagents like 3-(N,N-dimethylamino)propyl chloride. The API synthetic route do not involve use of nitrites or other nitrosating agents and thus risk of NDSRI formation during API synthesis is generally low. However nitrites from common excipients like microcrystalline cellulose, magnesium stearate etc. can lead to formation of the NDSRI impurities in Doxepin hydrochloride by interaction with the dimethylamine functional group on the API.
Key Takeaways:
Companies should perform a comprehensive risk assessment of potential for formation of Nitrosamines and NDSRI impurities in the drug product and take mitigation measures. This should include:
Leave a Comment
You must be logged in to post a comment.