USFDA Updates Consideration for CES for
Biosimilarity CAA more sensitive than CES The USFDA has published a draft guidance updating scientific

Warning letters, 483s, Recalls, Import Alerts, Audit observations
Biosimilarity CAA more sensitive than CES The USFDA has published a draft guidance updating scientific

USFDA has published over 200+ redacted Complete Response Letters (CRLs) issued to drug applicants. The
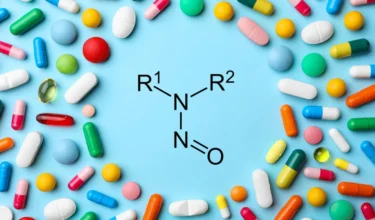
USFDA has allowed drug manufacturers additional time for submission of mitigation actions and required changes

USFDA has announced the Quality Management Maturity (QMM) Prototype Assessment Protocol Evaluation Program for the

FDA issued Warning letter to two Chinese API manufacturers following for cGMP violations including failing

USFDA is proposing to remove oral phenylephrine as an active ingredient in over-the-counter (OTC) drug

The USFDA has published a new guidance titled “Review of Drug Master Files in Advance

The guidance provides information on how FDA decides goal dates for a drug application (ANDA,

The USFDA released draft guidance on New Dietary Ingredient Notification (NDIN) Master Files for dietary

FDA has announced the over-the-counter (OTC) monograph drug facility (MDF) fee rates under the OTC