

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations





Authored by: Srinivas Churya
In the pharmaceutical industry, analytical division and analytical laboratories plays a significant role in ensuring the quality, safety, and efficacy of medicines. The laboratories reputation rests on its ability to deliver consistent and high-quality results. The accuracy, consistency and reliability of the laboratory results depends on various critical factors, including robust systems and procedures, samples handling and maintenance, calibration of instruments and accessories, qualification and validation, laboratory environment and conditions like temperature and humidity. However, a key factor in ensuring this reliability is the competency of the analysts themselves, their training and the process for qualifying the analyst’s as competent. And of course, the integrity of all the laboratory activities – data integrity and compliance is another essential factor for protecting interest of the patients as well as the laboratory.
Consistency and Quality in Analytical Results
Accuracy and consistency in lab results is essential, as it underpins the reliability and trustworthiness of the analytical results generated by the laboratory. The skills and competency of the analysts who conduct the testing is a key factor affecting the accuracy and consistency of results. This requires comprehensive and effective training of the analysts which focus on imparting the right skills.
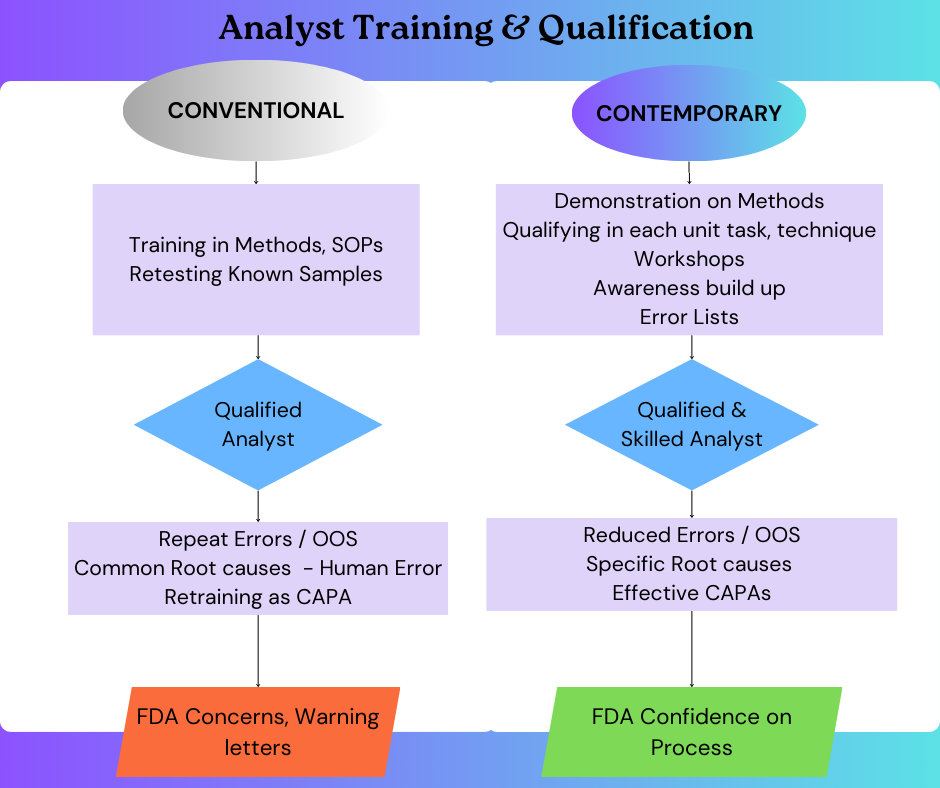
Conventional Approach to Training and Qualification
Traditionally, analyst training and qualification has been a relatively standardized process. Analysts typically undergo a few weeks of Standard Operating Procedure (SOP) reading followed by analysing previously tested samples to achieve results within acceptable limits. Typically, with acceptance criteria like +1% variation compared to the previously established results; the analyst will be certified as “Qualified”. Same approach is applied to both freshers and experienced analysts joining an organization. With this technically the SOP requirement of qualifying the analyst are met. While this approach helps in basic familiarity of the analyst with the different tests, it falls short in preparing analysts for real-world complexities and do not address the intricate skills required for accurate analytical work. This requires a more comprehensive and integrated training and qualification programme, one that considers the specific requirements of each type of analytical task.
Out of Specifications, Root causes & Human Error.
One critical focus area in all regulatory inspections, especially the FDA inspections is handling of Out of Specification (OOS) results. Human error in analysis is often cited as the primary cause for OOS results and OOS invalidated. However, regulatory bodies, notably the FDA, are increasingly sceptical of “human error” as a root cause. FDA Warning letters increasingly cite OOS investigations with root causes like laboratory error, analyst error, glassware contamination etc as inconclusive OOS investigations – paltry investigations and corrective actions (CAPA) inadequate. So now the Firms has to investigate the OOS incidents thoroughly before concluding root cause for laboratory errors– it could be procedural lapses, instrument-related issues or analyst related issues like poor analytical practices, poor analyst skills, analysts’ oversight, analysts handling multiple tasks at same time and so on. Appropriate CAPA need to be established for the identified root cause so that the OOS does not recur. To handle this pharma industry need well-trained, empowered and strong, confident analysts.
Comprehensive and Integrated Training and Analyst Qualification
Analytical tasks in a lab are diverse, and each analysis comes with a set of unit tasks or processes. For example:
For each of these activities or unit tasks, the qualification process should address individual unit tasks within the analytical process, with stringent acceptance criteria to qualify analyst for each task. Further the analyst shall also quality in specific analysis, performing qualification analyses in replicates—typically in triplicate—to demonstrate accuracy and consistency. This ensure that not only the analyst has an overall understanding of the analysis, the analyst is competent to perform each unit tasks precisely.
Practical Demonstrations
The training programme in analytical procedures and techniques shall include practical demonstrations of lab techniques, especially for freshers. For example:
Pipetting & Dilution Techniques
Weighing Techniques
Accurate use of pipettes
These techniques can be demonstrated practically or through demonstration videos. Hands on practice and demonstration of the techniques by analysts significantly help improve their skill, competency and confidence.
Emphasizing ALCOA++ Principles from Day One
Analysts must be trained in Data Integrity and ALCOA++ principles (Attributable, Legible, Contemporaneous, Original, Accurate, and the additional elements of Complete, Consistent, Enduring, and Available) from the beginning of their training. This ingrains data integrity into their work ethos and reinforces the meticulous care needed in every step of analysis and documentation.
Anticipating Potential Errors
Another essential part of the training is also providing analysts with a list of potential errors that can occur in each process or tasks involved in analysis. Discuss this with team of analysts, and encourage the team to discuss how such errors can be avoided. Regular discussions and team reviews can reinforce the learnings and skills learned, leading to continuous improvement in lab practices.
Conclusion
In conclusion, in pharmaceutical industry, the analyst qualification process is a key component for ensuring accuracy and quality of laboratory results. By adhering to the best practices and establishing rigorous standards for competency, analyst qualification significantly reduces the risk of human error, thereby enhancing the reliability of analytical results. Practical training with demonstration helps in learning the right analytical skills. The analyst qualification should not only focus in achieving matching results of qualification sample, but should qualify the analyst in each unit task involved in an analysis process. It enhances individual accountability of analysts, compliance and adherence to regulatory requirements. This significantly contributes to audit success and operational excellence of the organization
Leave a Comment
You must be logged in to post a comment.