CDSCO Flags Spurious Drugs of PAN D, Uri
CDSCO has published list of drugs declared as spurious in the month of September 2024.

Warning letters, 483s, Recalls, Import Alerts, Audit observations

CDSCO has published list of drugs declared as spurious in the month of September 2024.

Accord Healthcare Inc, US is recalling one lot of Cisplatin injection due to failed impurities.
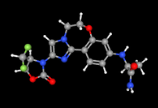
USFDA has approved Inavolisib ((Itovebi) of Genentech in combination with palbociclib and fulvestrant, for the

The European Medicines Agency (EMA) has initiated a review of finasteride and dutasteride due to

CDSCO has published an update on the Spurious and Misbranded drugs in September 2024. Medicinal

CDSCO has published alert of list of drugs Not of Standard Quality (NSQ) identified during

FDA has issued an Updated Guidance for Control of Nitrosamines in Human Drugs in September

Following the USFDA 483 issued in April 2024 and the OAI classification in July 2024,
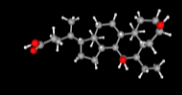
The European Commission (EC) revoked the conditional marketing authorization (CMA) of Advanz Pharma’s Ocaliva (obeticholic

CDSCO has published a drug alert regarding voluntary recall of Tisseel Lyo Fibrin Sealant VHS/D