

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations
USFDA has approved Checkpoint Therapeutics Cosibelimab-ipdl (Unloxcyt) for treatment of skin cancer CSCC (metastatic cutaneous squamous cell carcinoma).
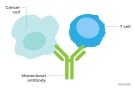
Cosibelimab-ipdl is a programmed death ligand-1 (PD-L1) blocking antibody. Unloxcyt dosage is 1200mg administered as an intravenous infusion every three weeks.
As per a press release by Checkpoint cSCC is the second most common type of skin cancer in the United States with annual incidence of approximately 1.8 million cases. cosibelimab-ipdl is a human immunoglobulin G1 (“IgG1”) monoclonal antibody that binds PD-L1 and blocks the interaction between PD-L1 and its T cell receptors, PD-1 and B7.1 thus releasing the inhibitory effects of PD-L1 on the anti-tumor immune response. Unloxcyt is the first PD-L1 inhibitor approved for treatment of CSCC while other approved drugs for the indication like Mercks Keytruda, Regeneron’s Libtayo are programmed death receptor-1 (PD-1) inhibitors.
Leave a Comment
You must be logged in to post a comment.