

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations
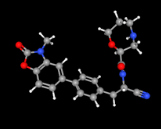
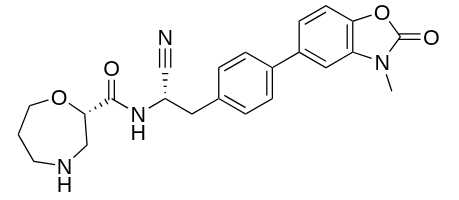
USFDA has approved Insmed’s Brinsupri (Brensocatib) for Bronchiectasis, a chronic drug disease. The medicinal product will be available as 10 and 25mg once daily tablets.
In a press release Insmed’s claimed that Brinsupri is the first and only treatment for Non-Cystic Fibrosis Bronchiectasis (NCFBE). NCFB is a chronic and progressive disease which can lead to permanent lung damage and lung function decline. Bronchiectasis causes permanent enlargement of airways of the lung, leading to inflammation, infection, chronic cough and mucus. BRINSUPRI is a first-in-class dipeptidyl peptidase 1 (DPP1) inhibitor, designed to inhibit the activation of NPS enzymes (neutrophil serine proteases) in neutrophils and directly targets neutrophilic inflammation.
Insmed’s has already applied for approval of Brinsupri in Europe and UK and planning to submit applications in Japan later this year.
Insmed’s Press Release on FDA approval of Brinsupri
Leave a Comment
You must be logged in to post a comment.