Controlled Correspondence / Scientific A
USFDA Controlled Correspondence Related to Generic Drug Development USFDA Guidance Document – Controlled Correspondence Related

Warning letters, 483s, Recalls, Import Alerts, Audit observations
USFDA Controlled Correspondence Related to Generic Drug Development USFDA Guidance Document – Controlled Correspondence Related
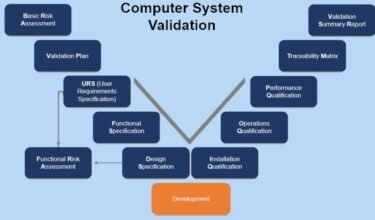
Qvents compilation of regulatory guidance’s on Computer System Validation, Electronic Data Review from USFDA, EMA,
APIC (Active Pharmaceutical Ingredients Committee): Guidance On Aspects Of Cleaning Validation In Active Pharmaceutical Ingredient
USFDA Sterile Drug Products Produced by Aseptic Processing — Current Good Manufacturing Practice – Guidance
WHO TRS 1025 – Annex 4: WHO Good Chromatography Practices EC (European Council) Directive 96/23/EC:
USFDA: Drug Master Files: Guidelines USFDA: Drug Master File (DMF) Templates USFDA: Drug Master Files