

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations
Elanco Animal Health announced that the USFDA has completed its comprehensive, multi-year review of Bovaer (3-NOP), a methane-reducing feed ingredient, and determined the product meets safety and efficacy requirements for use in lactating dairy cattle.
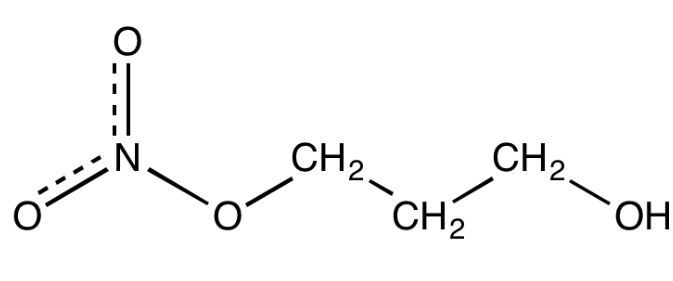
Bovaer with active ingredient 3-Nitroxy Propanol (3-NOP) was developed by DSM (now DSM-Firmenich). Elanco and DSM Firmenich have a strategic collaboration for registration, marketing and sales of Bovaer in US. Bovaer is already commercially available in 59 countries including the US, EU, UK, Canada, Mexico, Australia, most of Latin America.
Bovaer works by suppressing the enzyme in the cow’s rumen that forms methane. In a cow’s rumen, microbes help break down food and this releases hydrogen and carbon dioxide. An enzyme combines these gases to form methane. Bovaer suppresses the enzyme, so less methane gets generated. DSM claims just a quarter teaspoon of Bovaer per cow per day consistently reduces enteric methane emission by on average 30% for dairy cows and even higher percentages, on average 45%, for feedlot beef cattle.
References:
Leave a Comment
You must be logged in to post a comment.