

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations

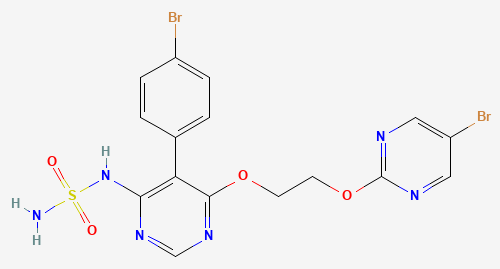
USFDA has approved Idorsia’s new drug Tryvio, a New Molecular Entity (NME) drug Aprocitentan for treatment of high blood pressure in patients who are not responding well to other medications. However, the approval comes with a black box warning, the most serious warning FDA gives to a drug due to risk of embryo-fetal toxicity (risk of birth defects). Women who are pregnant or plan to become pregnant should not take Tryvio. TRYVIO is available only through a restricted program (REMS) called the TRYVIO REMS. The recommended dosage of Tryvio is once daily oral tablet and it comes in a single strength of 12.5mg tablet
Idorsia announces USFDA approval of TRYVIO (aprocitentan) for treatment of blood pressure
Leave a Comment
You must be logged in to post a comment.