Qvents Apps and Tools For QMS and GMP Pr
Qvents provides useful tools for QMS processes – Risk Assessments, Continuous Process Validations, Supply Chain Traceability

Warning letters, 483s, Recalls, Import Alerts, Audit observations

Qvents provides useful tools for QMS processes – Risk Assessments, Continuous Process Validations, Supply Chain Traceability
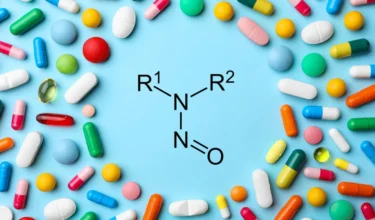
Qvents Apps & ToolsNitrosamine and NDSRI Risk Assessment Tools – APIs, Drug Products, Excipients, Water
As per EUGMP requirements, EudraLex, Annex 16: Certification by a Qualified Person and Batch Release,
Author: Venkiteswaran.T.K The USFDA’s 2011 guidance on process validation advocates a life cycle approach and

Qvents Apps & Tools Access the Excipient Risk Assessment Tool – Click Here According to