EMA To Review Finasteride and Dutasterid
The European Medicines Agency (EMA) has initiated a review of finasteride and dutasteride due to

Warning letters, 483s, Recalls, Import Alerts, Audit observations

The European Medicines Agency (EMA) has initiated a review of finasteride and dutasteride due to
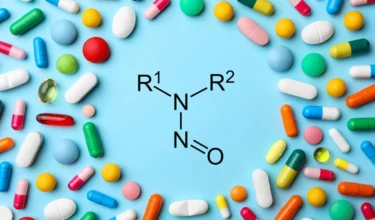
EMA(Europeans Medicines Agency) has updated the Appendix 1 Acceptable intakes established for N-nitrosamines with 15

Prospective applicants of Complex Generics (CG) can request for a joint meeting with FDA OGD

The GMP/GDP Inspectors Working Group coordinated by EMA has decided to continue the extension of

The European Commission has published the first Union list of critical medicines, together with the

EMA, the European Commission (EC) and the Heads of Medicines Agencies (HMA) are phasing out