

Discussion forum for Pharma Quality events, Regulatory Actions
Warning letters, 483s, Recalls, Import Alerts, Audit observations

Warning letters, 483s, Recalls, Import Alerts, Audit observations




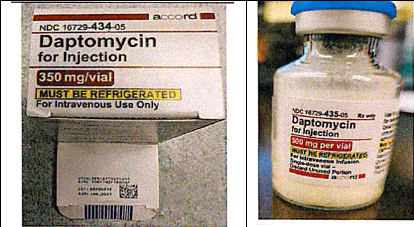
Accord healthcare recalled from US market Daptomycin for Injection 500 mg/vial and Daptomycin for Injection 350 mg/vial for labelling mixup. Recall followed a complaint that vials labelled as Daptomycin for Injection 500 mg/vial were found in cartons labeled as “Daptomycin for Injection 350 mg/vial. The product were manufactured by Intas Pharmaceuticals, India.
Accord Healthcare, Inc. is voluntarily recalling a single lot of Daptomycin for Injection 500 mg/vial, and Daptomycin for Injection 350 mg/vial product contained in cartons imprinted with lot # R2200232 Exp: 01/2025 to the consumer/user level.
Accord received a product complaint report from a hospital pharmacy that vials labeled as “Daptomycin for Injection 500 mg/vial” were found in cartons labeled as “Daptomycin for Injection 350 mg/vial”. The lot and expiration date printed on the outer carton and inner vial are the same and correspond to “Daptomycin for Injection 500 mg/vial.” Accordingly, Accord is voluntarily recalling all of lot #R2200232, Daptomycin for Injection 500 mg/vial, which may be in outer cartons that read “Daptomycin for Injection 500 mg/vial” OR “Daptomycin for Injection 350 mg/vial.”
The lot number, NDC number and expiration date of affected Daptomycin 500 mg/vial and Daptomycin 350 mg/vial product is shown in the table below:
Product | NDC | Lot number/Expiration Date |
Daptomycin for Injection 500 mg/vial | 16729-435-05 | R2200232, 01/2025 |
Daptomycin for Injection 350 mg/vial | 16729-434-05 |
It is typical to have reduced change over cleaning and line clearance requirements when different strengths of a product manufactured / packed in same line. Companies should evaluate, perform risk assessment and implement systems, procedures to prevent product mixups, with adequate line clearance procedures for change over, adequate batch /lot segregation, in process labelling, control on dispensing, issuance, handling of printed packing materials – labels, cartons, outer cartons.
Leave a Comment
You must be logged in to post a comment.